February 2026
In regulated industries, validation is not optional. A Risk-Based Validation Approach has emerged as a response to increasingly configurable, cloud-based, and continuously updated systems, where traditional one-size-fits-all validation methods struggle to keep up. As a result, many life sciences and regulated companies are adopting more focused and efficient validation practices that better reflect real operational risk.
Why do traditional validation models no longer fit modern systems?
Legacy validation frameworks were designed for static, on-premise systems with infrequent changes. Every function was tested to the same depth, documentation was extensive, and updates often triggered full re-validation cycles. While this approach offered structure, it also created heavy operational burdens, long release timelines, and unnecessary validation work on low-impact features.
Modern platforms such as cloud applications, SaaS solutions, and configurable enterprise systems evolve continuously. Applying identical validation effort to every component no longer reflects real operational risk. Regulators recognize this reality, which is why guidance increasingly emphasizes criticality, patient safety, data integrity, and product quality rather than exhaustive documentation for its own sake.
What does a risk-based validation mindset actually means?
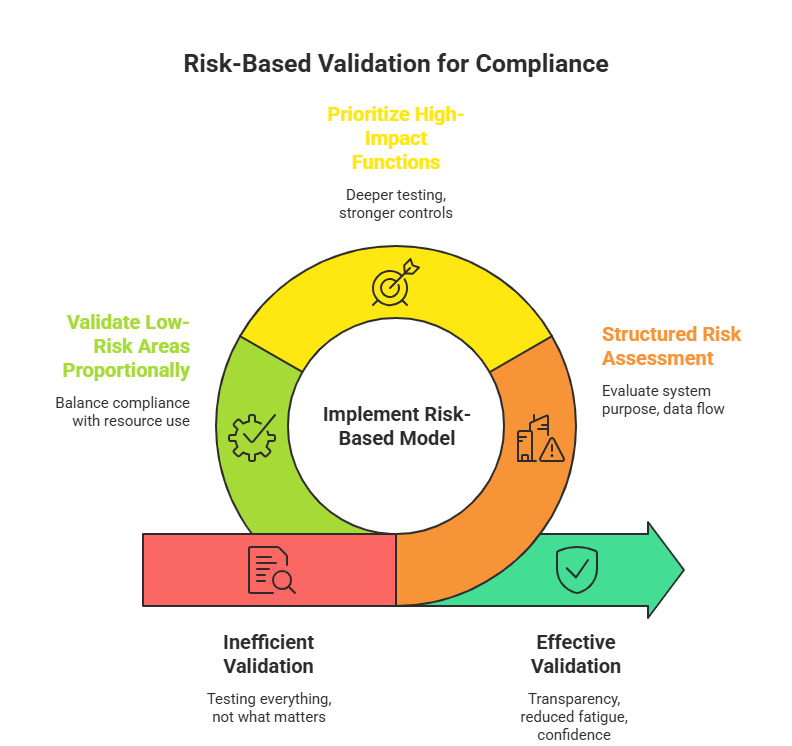
At its core, a risk-based model aligns validation effort with potential impact. Instead of asking “Did we test everything?”, organizations ask “Did we sufficiently control what matters most?”. This mindset prioritizes systems, processes, and functions based on their effect on regulated outcomes.
A structured risk assessment evaluates factors such as system purpose, data flow, user roles, and failure consequences. High-impact functions receive deeper testing and stronger controls, while low-risk areas are validated proportionally. This balance allows compliance without wasting time and resources.
When implemented correctly, the Risk-Based Validation Approach improves transparency, reduces validation fatigue, and strengthens confidence in system reliability.
How is risk-based validation of computer systems applied in practice?
Implementing risk-based validation of computer systems starts with understanding how a system supports regulated processes. Not all software used in a regulated environment requires the same level of scrutiny. Some systems directly affect product quality or patient safety, while others support administrative or reporting tasks.
A practical implementation typically includes:
- Defining system boundaries and intended use
- Identifying regulated functions and critical data
- Performing documented risk assessments
- Mapping risks to controls and test coverage
- Verifying that controls effectively mitigate identified risks
This approach ensures that validation activities are traceable, justified, and aligned with regulatory expectations, without unnecessary over documentation.
Risk assessment as the foundation of effective validation
Risk assessment is not a checkbox exercise. It is a living process that should be revisited as systems evolve. Changes in configuration, integrations, or usage patterns can introduce new risks that require updated controls or testing.
Effective risk assessments consider both technical and business perspectives. Input from quality, IT, system owners, and end users helps ensure risks are accurately identified and realistically mitigated. This cross-functional involvement is one of the reasons regulators view risk-based validation favorably when done correctly.
By continuously re-assessing risk, organizations maintain compliance even as systems change, instead of reacting only during audits.
Aligning validation effort with regulatory expectations
Regulatory agencies consistently emphasize that validation should be fit for purpose. They expect companies to understand their systems, justify their validation decisions, and demonstrate control over high-risk areas. Excessive testing of low-impact functions does not increase compliance confidence and can obscure what is truly important.
Using a structured model for risk-based validation of computer systems helps organizations clearly explain why certain areas were tested more rigorously than others. This clarity is especially valuable during inspections, where inspectors look for rationale, traceability, and evidence-based decision making rather than sheer document volume.
Documentation that supports, not burdens
One of the major benefits of a risk-driven strategy is smarter documentation. Instead of producing large volumes of generic validation documents, teams focus on records that demonstrate understanding, control, and accountability.
Well-structured risk assessments, test summaries, and traceability matrices often provide stronger compliance evidence than hundreds of pages of low-value scripts. This documentation is also easier to maintain over time, especially in systems with frequent updates.
Turning validation into a business advantage
When validation aligns with actual risk, organizations gain more than compliance. Release cycles accelerate, system owners gain clearer visibility into controls, and quality teams shift from document production to oversight and improvement.
The Risk-Based Validation Approach transforms validation from a bottleneck into a strategic process that supports innovation while protecting compliance. It enables companies to scale systems confidently without increasing regulatory exposure.
A smarter way forward with the right partner
Adopting a risk-driven validation strategy requires experience, structure, and the right tools. This is where companies like Validify add real value. With deep expertise in automated validation and regulated cloud platforms, Validify helps organizations implement practical, defensible validation frameworks that align with regulatory expectations while reducing manual effort.
If your organization is looking to modernize validation, streamline compliance, and gain confidence in evolving systems, working with Validify can turn validation into a controlled, efficient, and future-ready process.
Validify's Expertise
Validify is a risk and validation management platform designed to provide customers with an advanced tool for IT risk assessment and software validation. The platform was designed to automate a significant part of the risk assessment maintenance to support continuous validation process, as well as automated and customizable template-based validation document generation. Validify also provides a built-in connector for the Salesforce Platform, providing real-time status of your Salesforce compliance and identifying changes in your org automatically.
About the author

Ido Raz is the co-founder and CEO of Validify. Ido has extensive experience in providing solutions for organizations in the life sciences and other regulated industries. Ido led global technological and implementation teams, specializing in providing IT compliance and quality applications. He is an experienced compliance and cGMP professional and a cloud technology enthusiast.
Want to hear more or book a demo? Click here